QUALITY / ISO13485
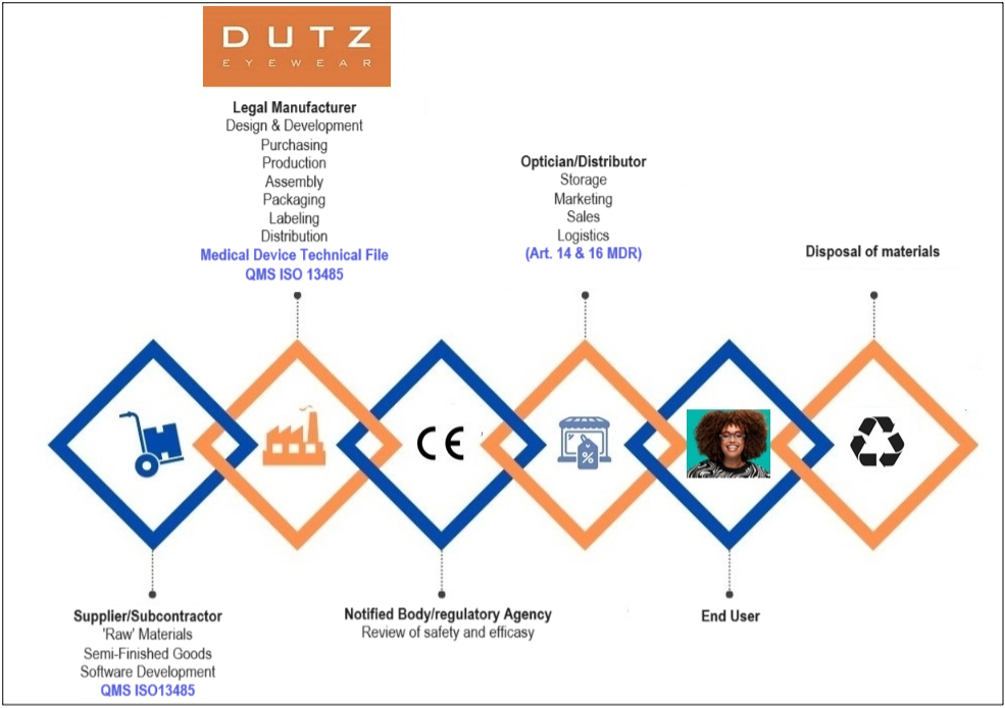
In compliance with the European Medical Device Regulation, we are proud to announce that – as the legal manufacturer of frames (an integral part of the ‘pair of glasses’/ i.e. the medical device), we have implemented a quality management system (QMS) meeting the requirements of the ISO 13485:2016 standard for medical devices.
On January 3 and 4, 2024, the certification body LRQA audited and approved our system. We are delighted to confirm that we have obtained the ISO 13485 certificate without any non-conformities!
Furthermore, our Technical File is complete, and we have registered our frames with the Ministry of Health in the Netherlands, providing comprehensive technical information on our designs, testing data and compliance with requirements.
As a result, Dutz Eyewear BV has fulfilled all conditions to apply the CE marking to our frames. Previously, our frames already bore the CE marking, compliant with old regulations. However, under the stricter European Regulation for Medical Devices, it is now mandatory to hold the status of Legal Manufacturer (LM) to affix the CE marking as a manufacturer. Opticians can identify this through stickers on the frames’ packaging bags. On the right you will find an example of the new Dutz (article) sticker and below a detailed explanation on the symbols used.
Symbol
Title
Description

Manufacturer
Indicates the manufacturer of the medical device.

Date of manufacture
To indicate the date on which a product was manufactured.

Batch code
To identify the manufacturer’s batch or lot code, for example on a medical device or the corresponding packaging.

Catalogue number
To identify the manufacturer’s catalogue number, for example on a medical device or the corresponding packaging.

Medical Device
Indicates the items is a medical device.

European conformity
When displayed on a product or its packaging, this signifies that the product complies with the applicable regulations within the European Economic Area (EEA), which includes the European Union, Switzerland, Liechtenstein, Norway, and Iceland. Additionally, the CE mark confirms that the manufacturer maintains control over the product and its associated processes throughout the entire lifespan of the frames
QUALITY
-
Designed in the Netherlands
We oversee the entire eyewear creation process from initial design to component production. This meticulous approach ensures a premium collection of frames that not only provide comfort but also boast a stylish appearance on any face. Our frames are crafted using allergy-friendly and durable stainless-steel materials, guaranteeing longevity and quality in design.
-
Natural materials
Our frames are crafted from premium acetates sourced from cellulose acetate, derived from cotton and wood. This material offers exceptional resistance and malleability, ensuring optimal comfort and durability.
-
Certified materials
Material selection is a crucial aspect of our creative process. We collaborate with esteemed suppliers from Italy, Germany, South Korea, and Japan, renowned for their expertise in delivering sophisticated, durable, and comfortable materials.
- STAINLESS STEEL FROM GERMANY
Our stainless steel is sourced from the German steel industry. Formulated with various alloys, it offers flexibility and resilience, ideal for intricate finishes and detailing. This hypoallergenic material minimizes skin reactions. - TITANIUM AND BETA-TITANIUM FROM JAPAN
Titanium and Beta-Titanium, lightweight and hypoallergenic materials from Japan, provide exceptional flexibility and strength. Pure titanium frames conform to diverse faces shapes, ensuring a sensation of utmost lightness, almost imperceptible.
- STAINLESS STEEL FROM GERMANY
-
Components
The hinges and core temples are manufactured by Comotec and OBE, prominent Italian and German companies in the industry. With the use of high-quality components, we ensure durability and resistance.